Customer Needs at the Centre of Patient Dynamics Development
Medaffcon develops the Patient Dynamics tool based on customer feedback and user experience.

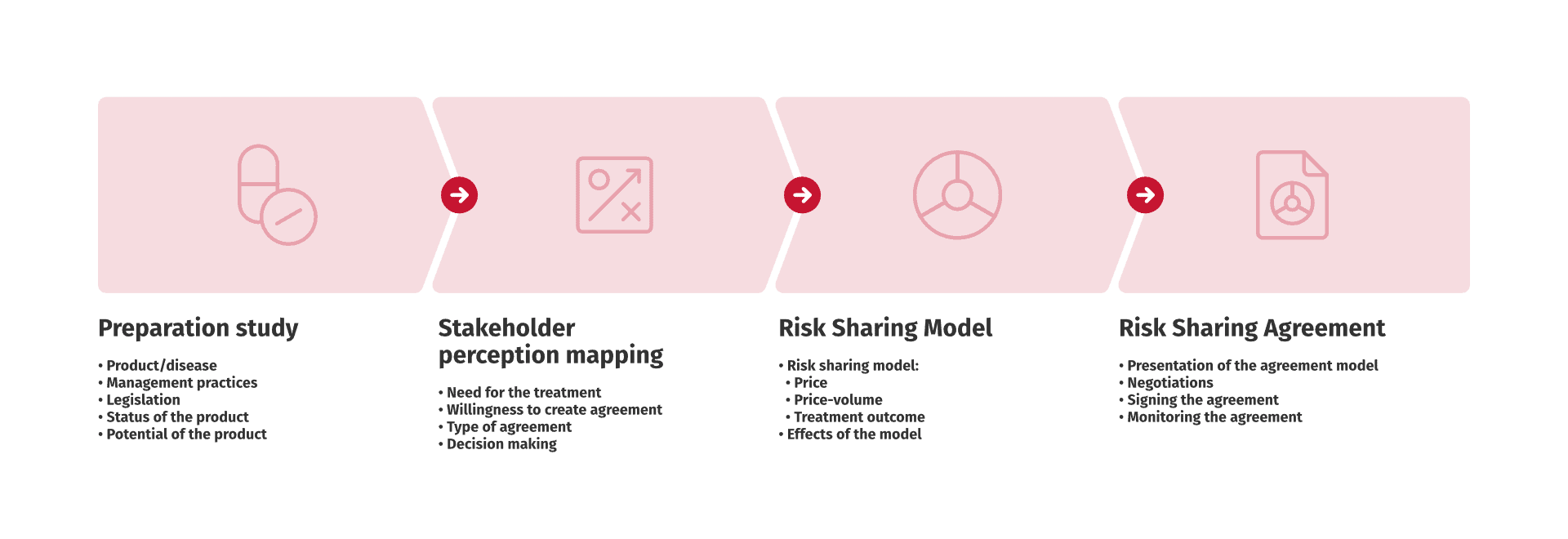
Comprehensive solutions for implementing risk-sharing models.
Confidential price agreements have become more widespread with regard to hospital medicines, while decisions on conditional reimbursement are becoming more established in the price and reimbursement processes of outpatient medicines.
Confidential agreements can be risk-sharing agreements (for example, pay for performance, conditional treatment continuation, or other contracts that reward the patient for the benefit of the treatment) or a method for sharing and managing uncertainty about the cost and effectiveness of medicines to the satisfaction of both parties. This will eventually make the medicine available to those patients who will benefit from it.
”Risk sharing agreements aim to solve the classic pharmaceutical sales dilemma of who bears the risk of effectiveness and cost effectiveness related to the purchasing and using of medicines.”
“We at Medaffcon execute risk-sharing agreements between the parties, ensuring that the agreements are operational and mutually beneficial. ”

Medaffcon develops the Patient Dynamics tool based on customer feedback and user experience.

Patient Dynamics tool compiles up-to-date and regional data on medicine use in Finland and Sweden.

The aim of the cooperation is to harmonize processes and produce joint assessments that each country can use in its own decision-making.

Principal Consultant
MSc (Economics)
+358 40 139 4001
jarmo.hahl@medaffcon.com
Jarmo joined Medaffcon as a partner and CEO in 2010, having previously worked in various expert and management positions in pharma companies for eight years. Jarmo has a degree in economics and before entering the pharmaceutical industry, he held an office at the Turku School of Economics and also worked as a research fellow at Turku University Hospital.
Jarmo has strong expertise in new health technology innovations and how the demands and expectations of authorities, markets and customers are matched. He also brings to Medaffcon extensive experience in health economics and its applications in research and commercialization, as well as a broad understanding of the ever-changing operating environment.
“From an access point of view, the Finnish health technology market is constantly becoming more demanding and therefore also more attractive from my point of view. On the other hand, increasing demands to demonstrate the effectiveness and cost-effectiveness of healthcare are broadening the scope and opportunities for Medaffcon to be involved in the development of healthcare as a whole”.