Multifunctional Real World Data
Real World Data stored in Finnish registries provides numerous opportunities. On this page, we introduce data sources, methods, and applications of Real World Data. The studies presented are parts of projects completed in cooperation with Medaffcon, our clients, and expert clinicians.
Where can Real World Data be utilised?
Real World Data (RWD) is stored in the social and healthcare registries during a patient’s treatment and life. Real World Evidence (RWE) drawn from RWD demonstrates the value of a treatment or a technology in treating patients. RWE may benefit healthcare service providers, authorities assessing therapies and pharmaceuticals as well as the society and patients.
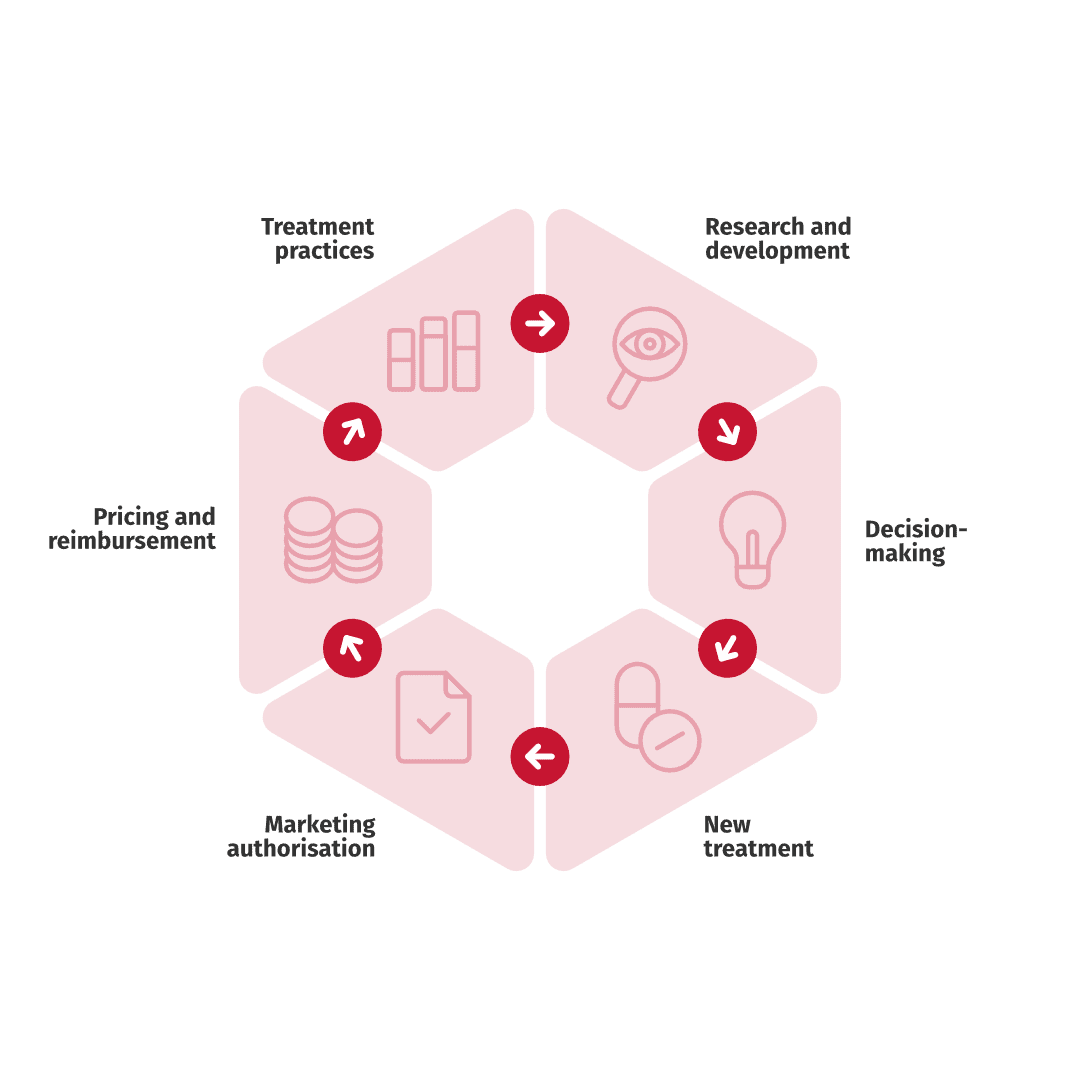
Local RWE, generated from local RWD, can be utilised in the different stages of a medicine’s life cycle –
from clinical trials to authority decision-making as well as in price and reimbursement negotiations. Local RWE describes treatment practises and patient population typical for a certain country such as Finland. However, the findings can be generalised, to other countries with similar healthcare systems, treatment practises, and patient populations.
The registry data allows examining, for example, the size, age, and mortality of a patient population or healthcare resource utilisation and its’ unit costs.
Why Finnish Real World Data?
Finnish RWD is important when local RWE is required in Finland. In addition, the Finnish health and social care registries are unique from an international perspective. Thus, RWD recorded in the Finnish registries is a strong option when weighing in which country research should be carried out in.

Finland has universal healthcare that is primarily taxation funded. Thus, all permanent residents in Finland (2022 population, 5.5 million) regardless of their social status or financial situation are entitled to equal public healthcare. Hence, the RWD in the nationwide Finnish social and healthcare registries covers all individuals living in Finland.
The information contained in the different registries can be linked by using a unique 11-digit personal identification number, which is given to every individual registered in the Finnish Population Information System.
Patient data has been stored in digital form in Finland for a long time. Longitudinal data enables extended retrospective follow-up of patients, which allows us to, for example, follow patients’ socioeconomic status or survival for ten years after getting an illness.
Most Finnish social and healthcare registers contain data from 2000 onwards, while others are much older. The Cancer Registry was established already in 1953, while Kela drug purchase reimbursement data is available from 1995 onwards, and THL primary health- care data from 2011 onwards.
The Act on the Secondary Use of Social and Health Data, i.e. the Secondary Act, entered into force in Finland in 2019. The Secondary Act was introduced to create uniform conditions for the secure use of social welfare and healthcare data or other related personal data. Social and Health Data Permit Authority Findata was established soon after the introduction of the Secondary Act. Findata is responsible for permit processes, data aggregation, and secure use of RWD.
Finnish registries include some unique data that is not available in other countries, including data on patients’ primary care and occupational healthcare. In this brochure we present examples of studies utilising sick leave data. In addition, Finnish hospital data lakes provide valuable data.
Indirect costs caused by a disease
Falling ill or getting a disease often results in impair- ment of working ability. Productivity loss caused by sick leaves and premature retirement are indirect costs of a disease that can be examined from the Finnish registries. Occupational healthcare service providers also record employees’ short sick leaves not covered by the National Social Insurance Agency’s registries.
In chronic diseases where a patient lives with a condition and is able to work, sick leaves are an important part of the disease burden. These sorts of diseases include, for example, migraine and osteoarthritis.
In studies applying RWD, we found out that migraine patients in occupational healthcare had almost a double and hip or knee osteoarthritis patients almost a triple number of sick leaves compared to the matched control patients. Data on the sick leaves of a patient group help us understand the disease burden and can provide additional information for clinicians to support their treatment decisions.
REFERENCES
- Korolainen MA., et al. Burden of migraine in Finland: health care resource use, sick-leaves and comorbidities in occupational health care. Journal of headache and pain, 2019.
- Summanen M., et al. The burden of hip and knee osteoarthritis in Finnish occupational healthcare. BMC Musculoskelet Disord, 2021.
Patient’s survival, mortality, and treatment
A patient’s survival, mortality, and treatment can be reviewed from RWD in a diversity of manners. Death is one evidence of disease burden. Sometimes, a patient’s survival becomes shorter after having a diagnosis.
In a study utilising the data lake of the Hospital District of Southwest Finland and national Finnish registries, the survival of patients with advanced cutaneous squamous cell carcinoma was 4.5 years after the diagnosis. In another study applying national registries, the survival of patients with multiple myeloma was 3.7 years after the diagnosis.
In a severe disease, one purpose of the patient’s treatment is to prolong survival. In the above- mentioned study, the survival of patients with multiple myeloma was observed to be longer with those who had the disease later in time. This is why the treatment of the patient population was interpreted to have improved over time.
REFERENCES
- Toppila I., et al. Characteristics and survival trends in Finnish multiple myeloma patients — a nationwide real-world evidence study. Annals of Hematology, 2021.
- Tuominen S., et al. Retrospective, Registry-based, Cohort Investigation of Clinical Outcomes in Patients with Cutaneous Squamous Cell Carcinoma and Basal Cell Carcinoma in Finland. Acta Derm Venereol, 2022.
How can rare diseases be studied?
Sometimes specific data on the number of patients or prevalence is lacking. With the help of registry and biobank data, it is possible to trace the number of patients with rare diseases. In addition, it is possible to review demographic and clinical background data, range of symptoms and genetics, therapies and interventions as well treatment results and survival in patients with rare diseases.
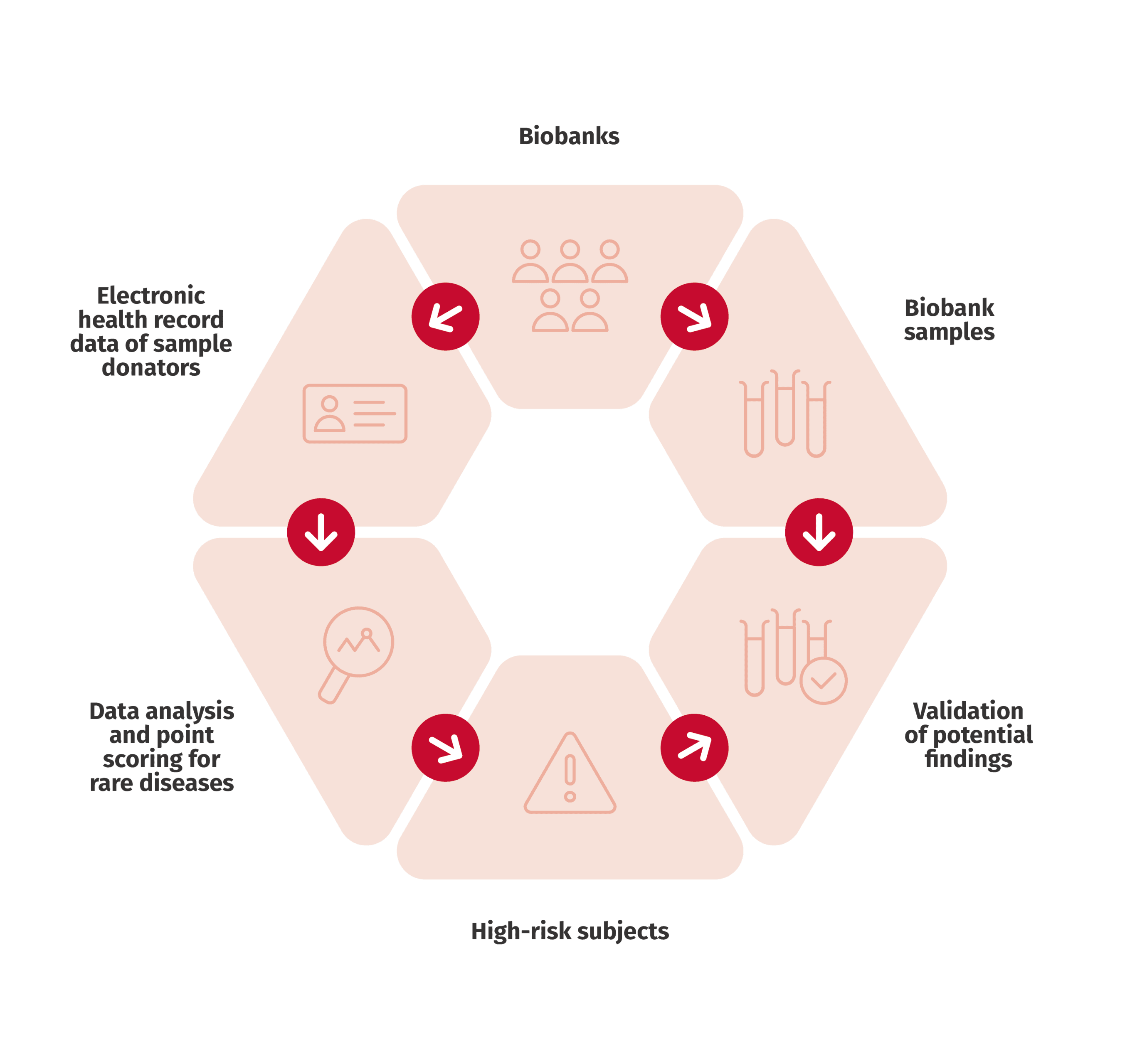
An example of our studies considering rare disease is Gaucher disease. Gaucher is a lysosomal storage disorder where the function of an enzyme that breaks down lipid molecules in the body cells is inhibited and lipids accumulate in the cell. The range of symptoms varies and includes symptoms that can be very common, complicating the making of a diagnosis. The prevalence of Gaucher diseases in Finland has been unclear. Our study reviewed the prevalence of Gaucher disease and developed methods to identify undiagnosed patients with rare diseases.
Another of our rare disease studies examined the prevalence and disease burden of pulmonary fibrosis as well as the identification methods of the disease in unstructured patient registry data. The study increased understanding of the progression of the disease and highlighted the importance of early diagnosis.
REFERENCES
- Lassenius MI., et al. Forced Vital Capacity (FVC) decline, mortality and healthcare resource utilization in idiopathic pulmonary fibrosis, European Clinical Respiratory Journal, 2019.
- Pehrsson M., et al. Screening for potential undiagnosed Gaucher disease patients: Utilisation of the Gaucher Earlier Diagnosis Consensus point-scoring system (GED-C PSS) in conjunction with electronic health record data, tissue specimens, and small nucleotide polymorphism (SNP) genotype data available in Finnish biobanks. Molecular Genetics and Metabolism Reports, in press.
- Savolainen MJ., et al. The Gaucher earlier diagnosis consensus point-scoring system (GED-C PSS): Evaluation of a prototype in Finnish Gaucher disease patients and feasibility of screening retrospective electronic health record data for the recognition of potential undiagnosed patients in Finland. Molecular Genetics and Metabolism Reports, 2021.
Treatment recommendations and real world treatment practices
Sometimes, patient’s treatment deviates from guideline-recommendations. This may be due to choices in clinical practise or those made by the patient themselves. Medication prescribed to the patient and medicines purchased by the patient can be compared utilising RWD.
Our study found deficits in the treatment practices of heart failure. Only half of the patients diagnosed with heart failure were measured with ejection fraction, although it is an essential diagnostic criteria for
heart failure. Half of the patients without the data, also lacked the records of the natriuretic peptide level, which in turn, is an important exclusion criterion for hearth failure. This demonstrates that the diagnosis of heart failure is often conducted through symptoms and findings. Diagnostic measurements are not always used.
Another of our studies observed that every fourth hospitalised patient with a cardiovascular event does not purchase prescribed lipid-lowering medicine from the pharmacy. Patients with cardiovascular events purchase their statins most diligently for approximately six months after the index event. Later, the use of medicine, especially high-dose statin treatment, decreases in many patients.
REFERENCES
- Huusko J., et al. Ukkonen H. Real-world clinical diagnostics of heart failure patients with reduced or preserved ejection fraction. ESC Heart Failure, 2020.
- Lassenius et al., ym. Cardiovascular event rates increase after each recurrence and associate with poor statin adherence. European Journal of Preventive Cardiology, 2020.
Registry data complements clinical trials
Randomised clinical trials have traditionally been held up as the gold standard for studying the efficacy of a treatment. The significance of RWD, however, increases constantly. A result of a clinical trial or RWE alone are more uncertain than when presented together. RWE confirms and complements data obtained from clinical trials.
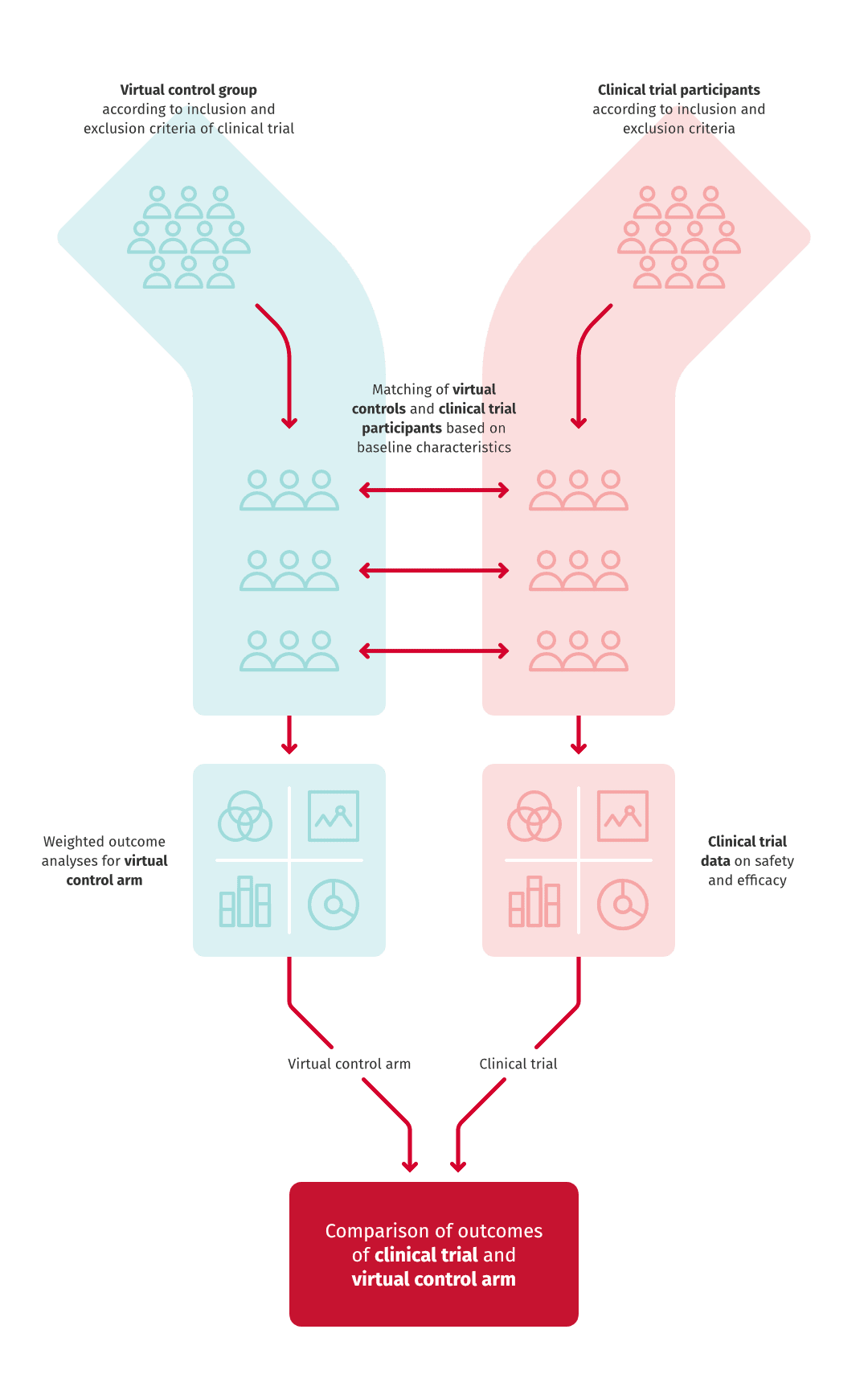
Instead of recruiting control patients to the trial, the control group of a clinical trial can be retrospectively described with registry data. This is called a virtual control arm, where the controls are collected from the registry by using the exclusion criteria of the patient selection for the clinical trial.
Typically, clinical trials last several years and cost millions of euros, whereas a virtual control study can be conducted in a year and with a fraction of the costs. In cancer treatments, the control arm of a clinical trial cannot usually be carried out because patients cannot be left without treatment.
RWD may complement clinical trial data also in other ways. An example of such is our study exploring the risk of arterial disease patients recurrent events. Because the settings of a clinical trial and RWE-study are different, their results may deviate from one another. In our study, the risk of death after recurrent cardiovascular event was larger than reported in earlier clinical trials. The result testifies of the necessity of studies utilising real world patient data. RWE studies include the entire patient population without limitations or control of circumstances, which means that they describe the function of a medicine or treatment in actual treatment practice.
REFERENCES
• Toppila I., et al. Cardiovascular event rate and death in high-risk secondary prevention patient cohort in Finland:
A registry study. Clinical Cardiology, 2022.
The potential of unstructured data
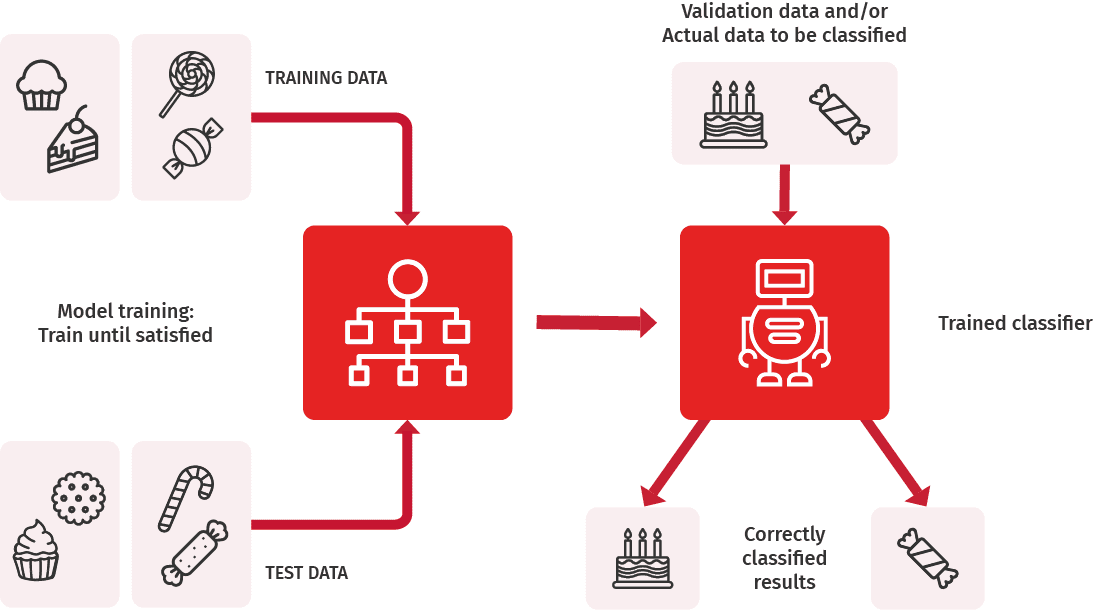
RWE studies generally use structured data, that is, data stored in registries in a tabular or some other standardised format. Electronic medical records, however, include lots of data stored in an unstructured form. Unstructured data is used in RWE research, but it includes challenges, starting from the collection of data. Machine learning has gained an important role in collecting unstructured data and its systematic transformation into structured format.
In some cases, patients cannot be included in a study only based on diagnoses recorded into the registry. One example of such studies is idiopathic pulmonary fibrosis, in which we were able to identify patients with the disease from unstructured data by using medical imagining and patient texts as well as pathologists’ statements in addition to entries of diagnoses.
Further, the smoking status of a patient is often an interesting background data in a RWE study, but it is rarely accessible in a structured format. In one study, we developed a machine learning algorithm to classify statements from patient records based on smoking status.
The developed algorithm classified the statements with excellent precision and thus exemplified the possibilities of current machine learning methods in achieving precise results.
REFERENCES
- Hölsä O. Machine learning-based classification of clinical cotes to extract smoking status from electronic health records. Master’s Thesis, Aalto University, Espoo, Finland, 2022.
- Lassenius MI., et al. Forced Vital Capacity (FVC) decline, mortality and healthcare resource utilization in idiopathic pulmonary fibrosis, European Clinical Respiratory Journal, 2019.
Expert solution & fresh perspectives
Medaffcon provides individually tailored Real World Evidence (RWE) research services in Finland Sweden and the Nordic countries. Our extensive expertise enables, alongside RWE research, other services utilising the same data, as you also have available our Market Access and Medical teams.
Let’s plan together a solution that meets your needs!
