Medaffcon Presented Insights from Finnish Real-World Data at European Lung Cancer Congress
Medaffcon's European Lung Cancer Congress (ELCC) poster showcased key findings from a recent study on non-small cell lung cancer (NSCLC).

We provide individually tailored Real World Evidence services to different industry and healthcare actors.
We provide individually tailored Real World Evidence services to different industry and healthcare actors in the Nordics. Our scientific experts and data scientists ensure that the correct research methods and data sources are used to answer your questions.
Real World Evidence (RWE) can be used to demonstrate the value of a certain treatment or technology for patients, healthcare service providers and society. Due to comprehensive healthcare, data in digital format, a personal ID, and progressive research legislation, Finland and Sweden are true model countries for Real World Evidence studies.

Medaffcon experts have solid experience from all life cycle stages of a product, whether it is communication towards payers, showing the clinical value or demonstrating the impact through data-driven value tools. Our broad expertise is at your service when planning and executing real world evidence studies and strategies. We identify the correct data sources and analysis methods to support your evidence need. Click each topic for more information.
We offer strategic Real World Evidence (RWE) input throughout the lifecycle of your product identifying data gaps and the best opportunities to show value, whether for payer negotiations, clinical decision making or post-marketing real world effectiveness. We help you stay up to date with your evidence generation strategy to provide you with the best tools for success.
We offer our customers Real World Evidence (RWE) workshops where we together define data gaps and needs in selected therapeutical areas or products and generate a real world evidence plan.
We offer our customers comprehensive services to generate Real World Evidence (RWE), including all aspects from (pre-)planning to publication and beyond. The services include:
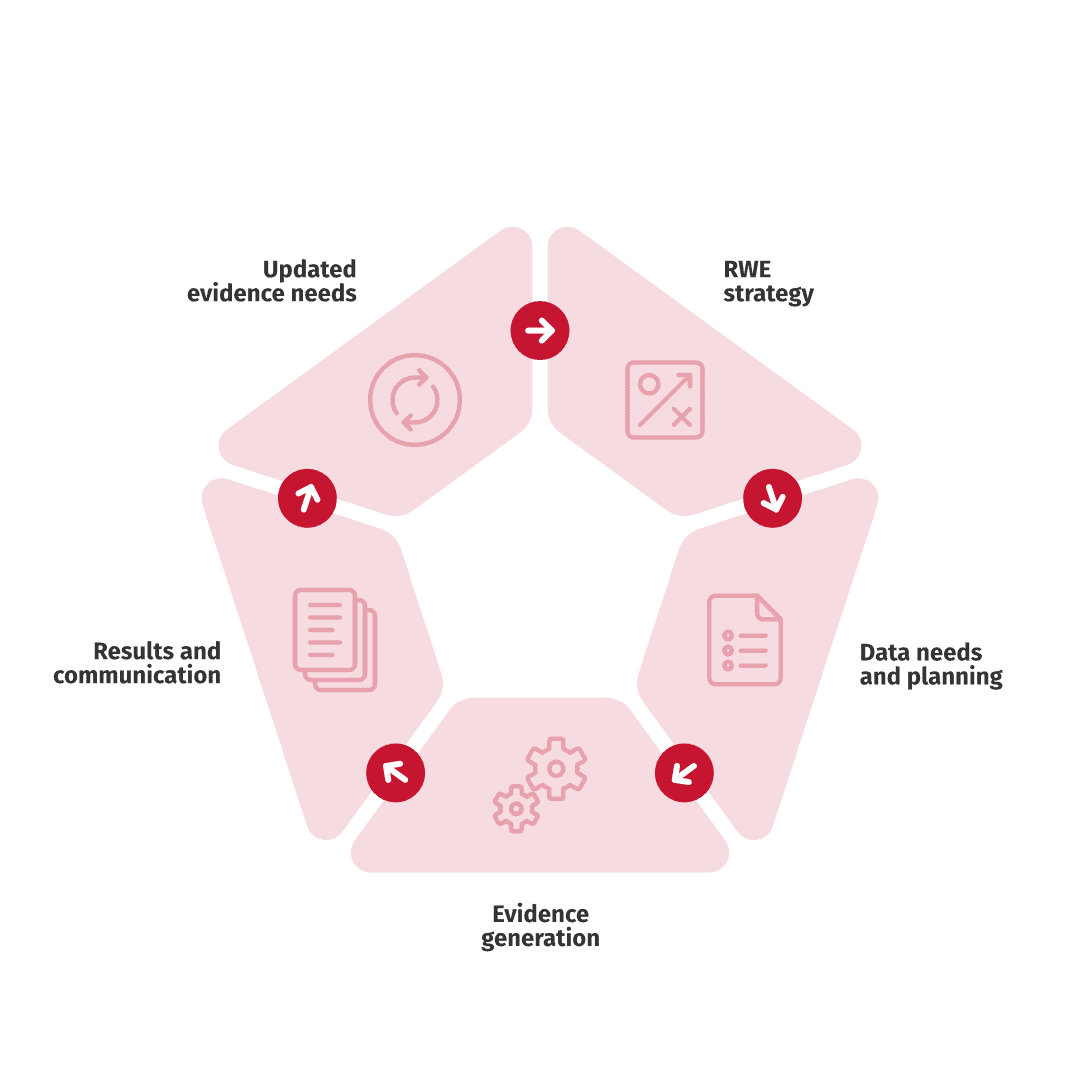
A full-scale Real World Evidence study is not always the optimal solution, both cost and timewise. For light and defined data needs, FastTrack requests may be the better approach. If you have a specific data need in mind, please contact us to plan the opportunities and discuss strategies.
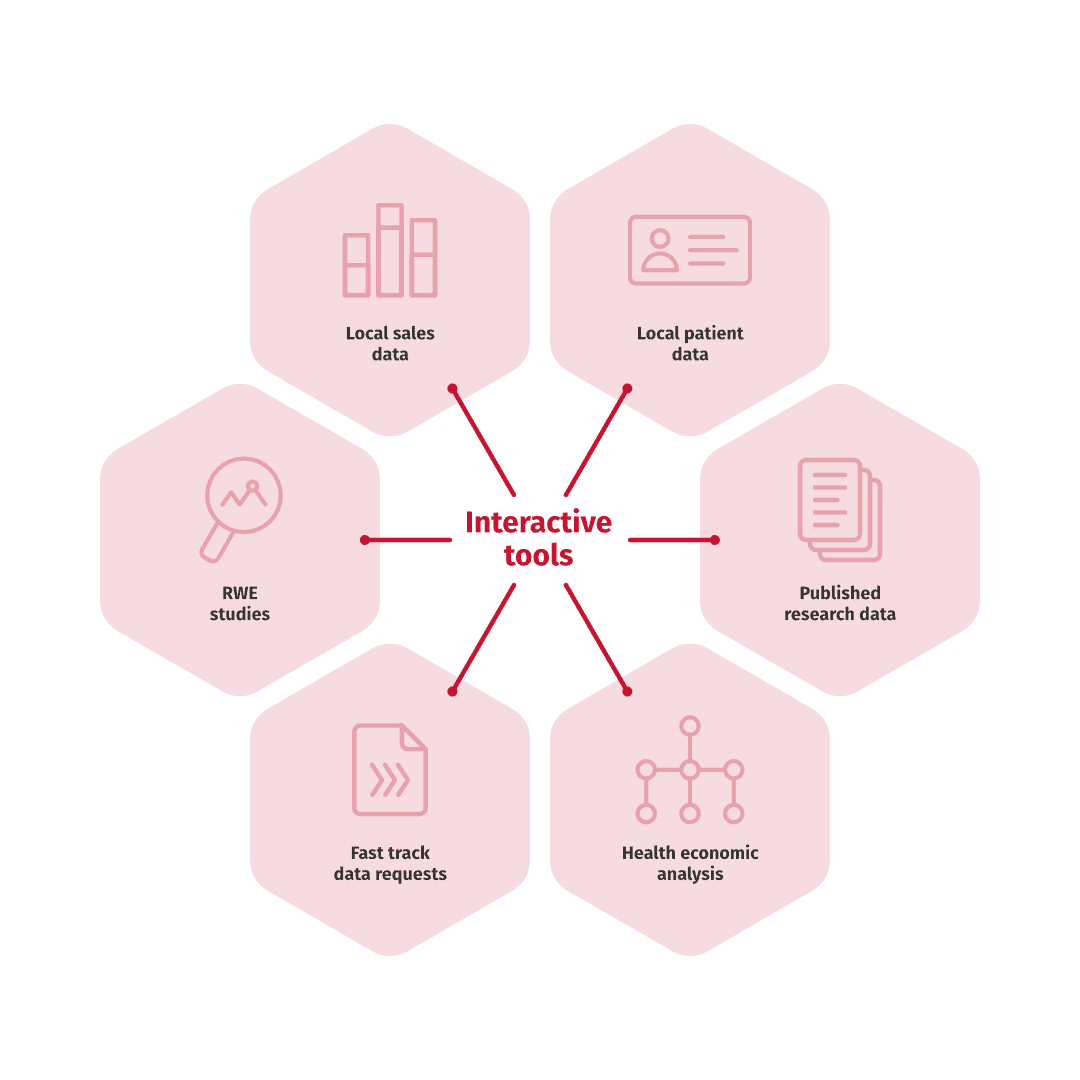
Data from various sources, including RWE studies, clinical trials and sales data, can be processed to a user-friendly format and used in communication with internal and external stakeholders. We help you make the most out of your data for informed decision making, utilizing our interactive value tools.
Real world data is often used in health economic analyses to support market access and health care decision making. Our experts support your evidence generation process from defining the research question to generating health economic evidence for payer and decision-maker discussions.
Read more about our Health Economic Modelling and Pricing and Reimbursement services.
Medaffcon is the leading expert in real-world evidence (RWE) and data analytics in the Nordics, with the largest team of RWE and data specialists in the region. We bring unparalleled experience in analyzing and leveraging RWE, having successfully delivered over 100 RWE projects since 2013.
Our RWE studies are closely related to health economic perspectives on interventions and clinical outcomes, providing valuable insights to support decision-making and improve healthcare interventions.
Since 2023, Medaffcon has expanded its footprint with offices in both Finland and Sweden, offering comprehensive Nordic services across the region.
Find examples of data sources, methods and applications used in our RWE studies here!
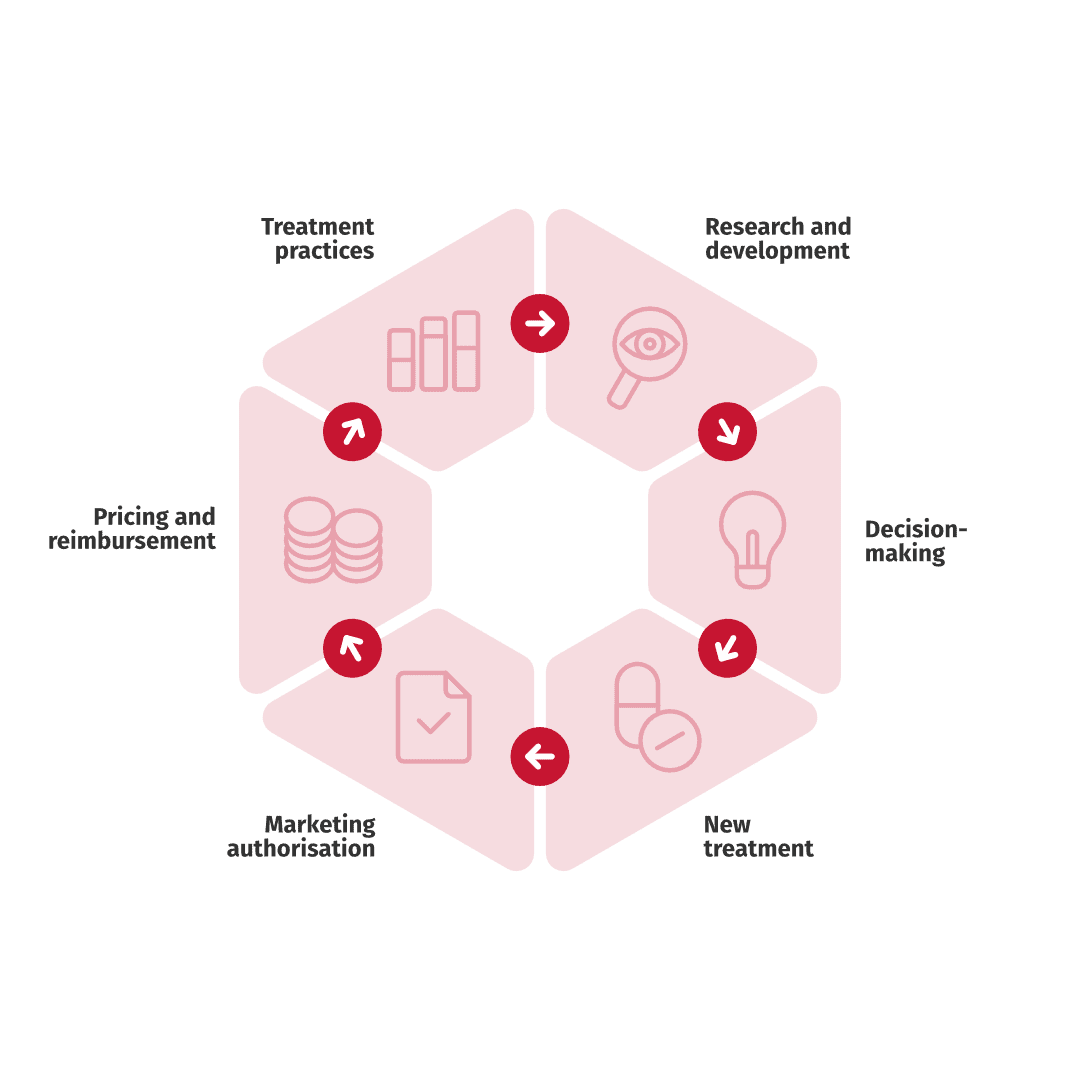
Real World Evidence can be used to understand patient populations, treatments and outcomes and the standard of care to assist clinical decision making. Increasingly, payers want evidence on current care, disease burden and treatment effectiveness in a real world patient population. Below are some typical settings where RWE studies are valuable.
FDA: Real World Evidence – Role in health care decisions
ISPOR: Real World Evidence – Improving standards and practice
Local data on patient numbers, outcomes, treatments and healthcare resource utilization are crucial aspects for cost-effectiveness and all stakeholders, especially the payer. This data is increasingly being utilized in budget impact and cost-effectiveness analyses to reduce the ever present uncertainty in health economic analyses.
In addition, showing the effectiveness in real life after the medication’s market entry is also becoming increasingly important. Real World Evidence studies can readily assess these topics.
Information on incidence and prevalence, as well as the survival of patients and patient subgroups, may sound trivial, but often there is a lack of recent data on these. Patient numbers are of significance for the healthcare system when planning care, for the payer to assess the budget impact and for the marketing company when planning new launches.
Real World Evidence is highly appreciated and often mandatory in negotiations with payers. Further, current treatment and outcomes and questions on healthcare resource utilisation can be assessed with Real World Evidence studies or FastTrack requests.
Data from RWE publications and other sources can also be used in discussions with decision makers and can be processed to a user-friendly format utilizing our interactive value tools.
Standard of care and outcomes are essential to understand when a new product enters the market, both from a clinical and payer perspective. Especially oncology drugs and treatments can enter the market with phase 2 data as clinical evidence.
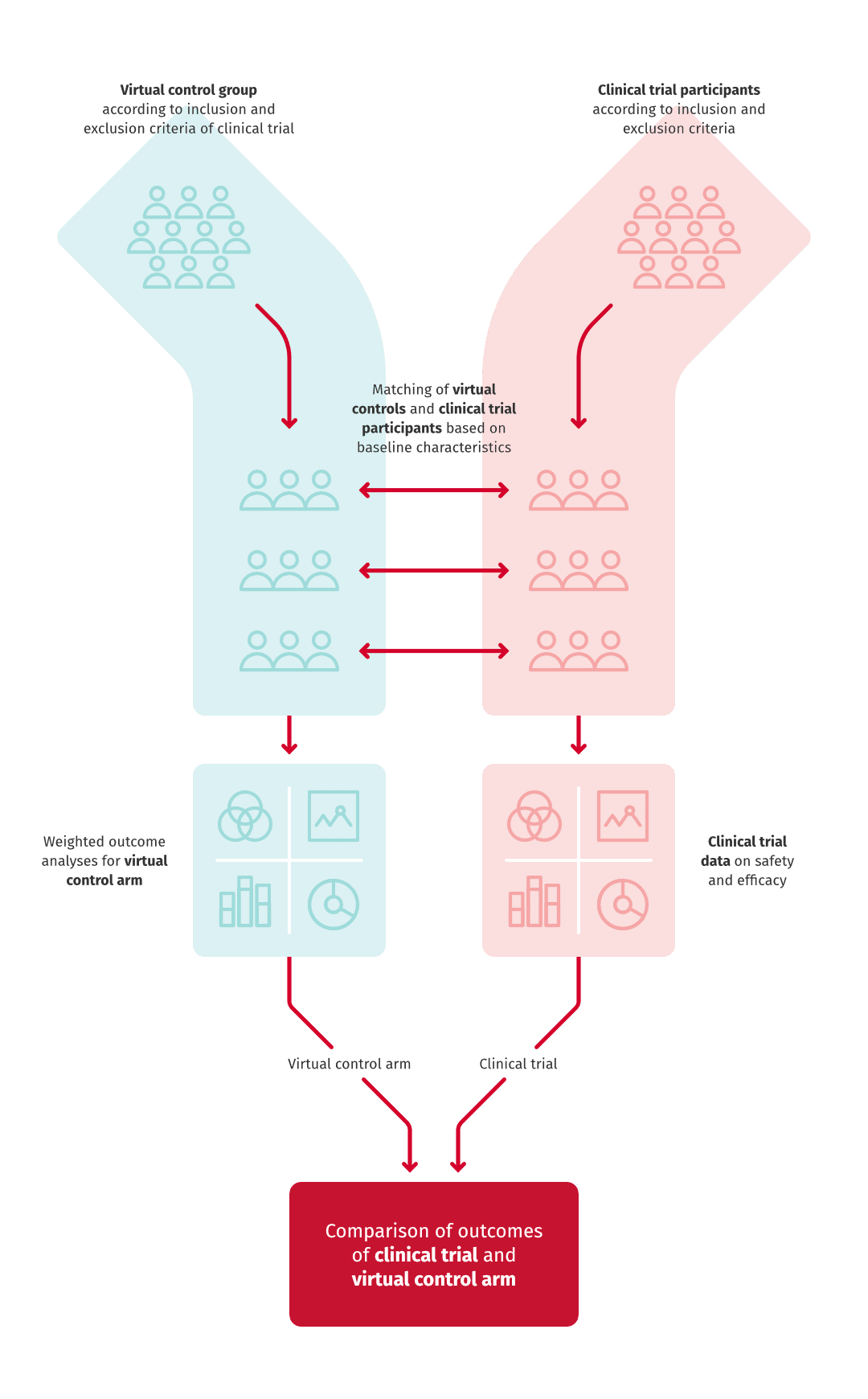
A control group for a clinical trial can be generated from retrospective registry data. This control group is called a virtual control arm. Patients in the virtual control arm are selected from registry data using the selection criteria of the clinical trial. Virtual control arms are used to supplement one and two armed clinical trials with Real World Evidence.
In some cases virtual control groups are formed because a clinical trial would be unethical. For example, in the case of cancer medicines patients cannot be left without treatment. Virtual control arms aim to act as comparators receiving standard of care when assessing the same outcomes as in the patients treated in clinical trials.
We at Medaffcon have conducted successful virtual control arm studies. Contact us, if you want to discuss more!
Patients transition between primary and specialty care, with treatment pathways often being complex and varying by region. By assessing treatment pathways through studies— including those in occupational healthcare— it is possible to gain a comprehensive understanding of regional differences and identify optimal approaches to care delivery.
Real-World Evidence (RWE) studies can help define patient subgroups, identify those who may benefit the most from specific treatments, and detect groups requiring more intensive monitoring. These insights can play a crucial role in optimizing healthcare resource allocation.
Regional differences in healthcare utilization and patient outcomes, incidence and prevalence, can be studied across the Nordics to gain insight into how care is organized.
Medaffcon’s RWE studies have been featured on numerous scientific publications across different specialty fields. Read summaries of some of our studies here and check out our publications here.
Interactive value tools combine published research data, local patient- and sales data and health economic analysis into one easy-to-use tool, especially popular in the customer interface.
After a treatment has become available for patients, information on patient adherence and treatment persistence is important. These aspects can be assessed in Real World Evidence studies to guide treatment and evaluate the impact on real world effectiveness.
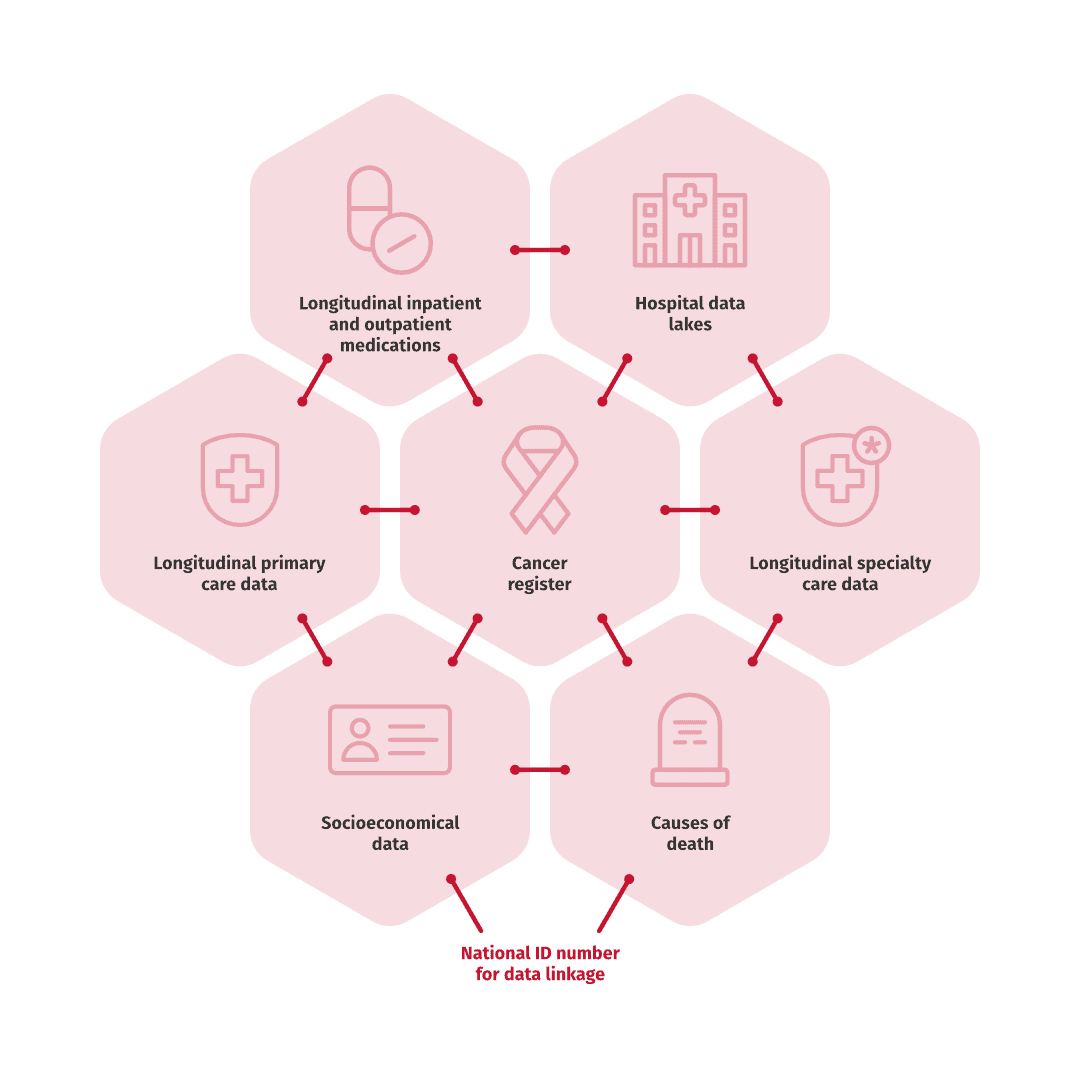
The Nordics have some unique Real World Data opportunities, with a base in a long tradition of longitudinal data from electronic medical records. Many data sources are national, covering the whole population, whereas in-depth analyses on hospital administered drugs and outcomes, can be studied at regional data lakes. The different data sources can be combined utilizing a unique personal identification number. Our scientific experts and data scientists ensure that the correct research methods and data sources are used to answer your questions.
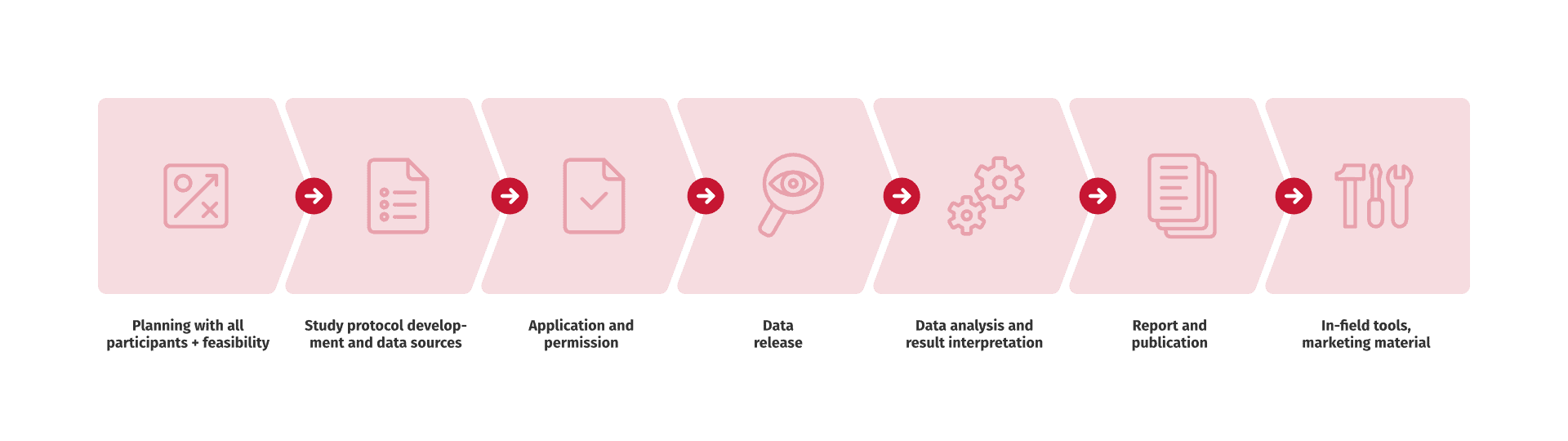
We help you identify the relevant data sources and plan the whole study together with you from protocol development and submission to authorities to data analysis, reporting, publication and beyond.
Medaffcon’s RWE studies have been featured on numerous scientific publications across different specialty fields. Read summaries of some of our studies here and check out our publications here.

Medaffcon's European Lung Cancer Congress (ELCC) poster showcased key findings from a recent study on non-small cell lung cancer (NSCLC).

Johan Rehnberg started working as a Scientific Advisor at Medaffcon’s Swedish office in August 2024. He is a dynamic researcher who values opportunities to learn new things and develop his skills – opportunities that Medaffcon provides.

The algorithm was originally developed to extract smoking status from patient texts with purpose to analyze the effects of smoking on postoperative complications. Today, it is also being utilized in lung cancer research.

Sr. Scientific Advisor
RWE Lead
PhD
+358 50 345 2393
mariann.lassenius@medaffcon.com
Mariann joined Medaffcon’s team in 2016 after finishing her PhD. The transition to real world evidence (RWE) research was a natural continuum to her previous research career. Through RWE studies, she has had the privilege to gain a broad insight into working with different stakeholders within the healthcare field. The vast proportion of her days goes towards interacting with clients, planning and performing RWE studies, and supporting Medaffcon’s RWE team. Subjects that keep her work interesting are the vast variability of customers and projects, problem-solving, and interacting with people.
“The number of RWE studies has increased since stakeholders within the healthcare industry have an increasing demand for knowledge-based decision making tools that need to be fulfilled. The future, therefore, has an ever-increasing emphasis on RWE”.

Biostatistician
Data Analysis Lead
MSc (Tech.)
+358 44 314 1597
iiro.toppila@medaffcon.com
Iiro joined Medaffcon in March 2017 as a Biostatistician. For the preceding four years, he has worked as a research assistant in an academic study group, analyzing clinical and genetic patient data. Iiro holds a Master of Science degree in Technology in Bioinformation Technology.
Iiro’s strengths include his strong expertise in statistics and data-analysis, hands-on experience in working with sensitive patient data, and strong interdisciplinary communication skills with experts from various fields. In the field, he is particularly interested in the large data amounts made available with the revolution of technology and how the information received such data can potentially be utilized to draw concrete conclusions, both in order to understand the nature of diseases and to advance the goals of the pharmaceutical industry and patient treatment.
“Machine learning and AI-based solutions will have a major impact on the healthcare sector now and in the future. However, effectively utilizing the already collected and available health-data will have a higher importance in order to improve health-care”.

Country Director Sweden
M.Sc (Econ.) & M.Sc (Health Econ)
+46 73 447 47 27
lisse-lotte.hermansson@medaffcon.se
Lisse-Lotte started at Medaffcon 1st of October 2024. Previously she was at a Swedish-German company as CSO Chief Scientific Officer, consulting European companies about Nordic health data opportunities and market access. She has a M.Sc (Econ.) from Helsingin School of Economics and a M.Sc (Health Econ) from Karolinska. Additionally a Ph.D student at the University of Turku in Health Economics. She has obtained a long experience from global pharma and medtech. She has lived over 20 years in Sweden.
The current development gives new possibilities to utilise data. With AI we can produce synthetic data and build digital twins that can actually support drug development and support healthcare providers. Innovative solutions are only useful if they are adopted to daily practice.
Old ways of working will vanish and RWD will be acknowledged as an excellent option or support for RCTs. As RWD is enabling more cost-effective evidence generation for new treatments. Treatments need to be more personalised so that the right drugs, diagnostics and devices are used for the right patients at the right time.